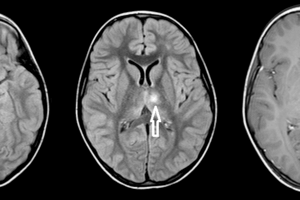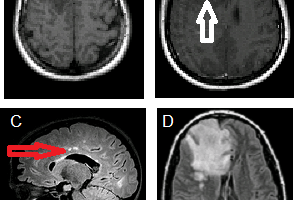
“Multiple sclerosis (MS) does not really remit” was the opening theme of this weekend’s conference. Lead by three MS-experts, Drs. Omar Khan, Guy Buckle, and Stephen Krieger, a roomful of neurology residents reviewed the epidemiology, classification, immunopathogenesis, disease course and their impact on therapeutic advancement.
MS does not remit – insights from pathology
As MS does not really remit, one cannot wait until visible disease manifestations to treat. Over the last 15 years, MS has been recognized as inflammatory and degenerative.

Antigens present (via the two largest immunologic organs of skin and GI) to the peripheral lymph organs. T-regulatory cells, which make up <1% of immune cells, control the vast majority of pathology in auto-reactive phenomenon, which results in diseases like MS, CIDP, and myasthenia. One key question in MS remains – how does the auto-reactive pathology target the CNS in MS?
Despite the predominance of pro-inflammatory cytokine activity in MS, decades of research to date shows the crosstalk makes it impossible to target any specific cytokine.
The four oldest MS treatments (interferons, glatiramer mitoxantrone, and natalizumab) act on T-cell pathways, but there is evidence for B-cell involvement: oligoclonal bands in CSF are in fact immunoglobulins, which are produced by plasma cells, which are mature B-cells. Meningeal B-cell follicles in secondary progressive MS are associated with early disease onset and severe cortical pathology (Magliozzi et al. 2007). Although these inflammatory cells go to the sub-pial layer, there is no MRI enhancement because the cells inhabit mere microns, about 1/1000 the tip of a pin, far beyond current MRI resolution.
Clinical pearls for MS care
Dr. Guy Buckle then transitioned to some clinical pearls, including that he tells patients not to call him in the middle of the night: MS attacks (aka exacerbation or relapse) must last at least 24 hours. Additionally, the definition includes sudden worsening of any MS symptoms or appearance of a new symptom that is separated from a prior attack by at least 1 month and is not due to environmental, metabolic, or infectious processes.
The natural history of MS without treatment was chronicled in a population under the Canadian health system prior to the advent of interferon-beta in 1993 (Weinshenker et al. 1989). Around 25% of patients never lose ability to perform ADLs, but ~ 15% become severely disabled in a short time (based on median time to reach EDSS of 6 at 15 years and of 8 at 46 years).
Understanding MS trials – why no placebo arms?
In examining trials design and outcome, one should look at how the controls, behavior of the placebo group, dropout rate (in MS trials, the dropouts are usually not replaced), and escape criteria from the study as the disease progresses. Placebo arms are unethical in the US and other countries that have the same FDA-approved MS treatments; this limits placebo studies to Eastern European and other countries, where MS patient have access to no treatment. Regarding the behavior of placebo groups, it is important as absolute relapse rate and reduction is a ratio of absolute reduction versus placebo group relapse rate.
Most phase II studies use MRI data, however EDSS is the gold standard for primary outcome endpoints of disease progression. Trial duration is usually 2 years and progression is confirmed in 3 or 6 months. Due to limited numbers of study patients, as in secondary progressive MS, 2 years may not even be long enough.
Returning to the opening point, evidence suggests that degenerative changes can occur in normal-appearing white matter and MS may be active in absence of clinical symptoms. Thus early disease-modifying treatment may slow the accrual of damage. (Ruiz-Peña et al. 2004. De Stefano et al. 2001. Filippi et al. 2003.)
Disclosures
A complete summary of “The Implications of Multiple Sclerosis Evidence in Changing Clinical Practice,” PRIME GME Today Advance Practice Conference. Attendance sponsored by independent educational grant from Biogen Idec.today’s conference can be found at the online GME Today Curriculum.
References
- Magliozzi R, Howell O, Vora A, Serafini B, Nicholas R, Puopolo M, Reynolds R, Aloisi F. Meningeal B-cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain. 2007 Apr;130(Pt 4):1089-104.
- Weinshenker BG, Bass B, Rice GP, Noseworthy J, Carriere W, Baskerville J, Ebers GC. The natural history of multiple sclerosis: a geographically based study. I. Clinical course and disability. Brain. 1989 Feb;112 ( Pt 1):133-46.
- Weinshenker BG, Bass B, Rice GP, Noseworthy J, Carriere W, Baskerville J, Ebers GC. The natural history of multiple sclerosis: a geographically based study. 2. Predictive value of the early clinical course. Brain. 1989 Dec;112 ( Pt 6):1419-28.
- Ruiz-Peña JL, Piñero P, Sellers G, Argente J, Casado A, Foronda J, Uclés A, Izquierdo G. Magnetic resonance spectroscopy of normal appearing white matter in early relapsing-remitting multiple sclerosis: correlations between disability and spectroscopy. BMC Neurol. 2004 Jun 10;4:8.
- De Stefano N, Narayanan S, Francis GS, Arnaoutelis R, Tartaglia MC, Antel JP, Matthews PM, Arnold DL. Evidence of axonal damage in the early stages of multiple sclerosis and its relevance to disability. Arch Neurol. 2001 Jan;58(1):65-70.
- Filippi M, Bozzali M, Rovaris M, Gonen O, Kesavadas C, Ghezzi A, Martinelli V, Grossman RI, Scotti G, Comi G, Falini A. Evidence for widespread axonal damage at the earliest clinical stage of multiple sclerosis. Brain. 2003 Feb;126(Pt 2):433-7.