Radiotherapy is commonly used as an adjuvant therapy to surgery and chemotherapy in the management of primary brain neoplasms. Radiation destroys cells and damages the tumor blood supply. Addition of radiation therapy to surgery has been shown to improve GBM survival from 3-7 to 7-12 months.
Diagnosis: Radiation necrosis

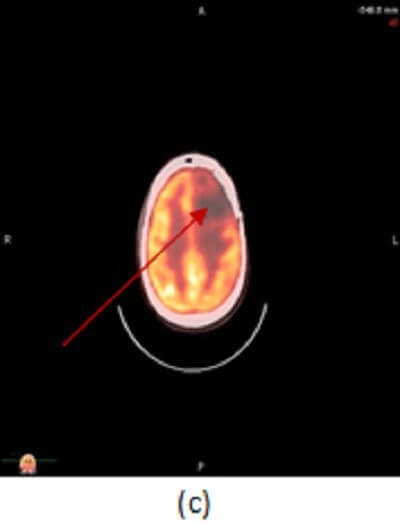
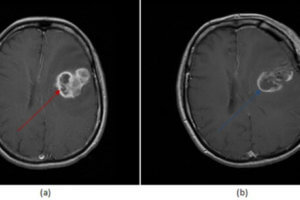
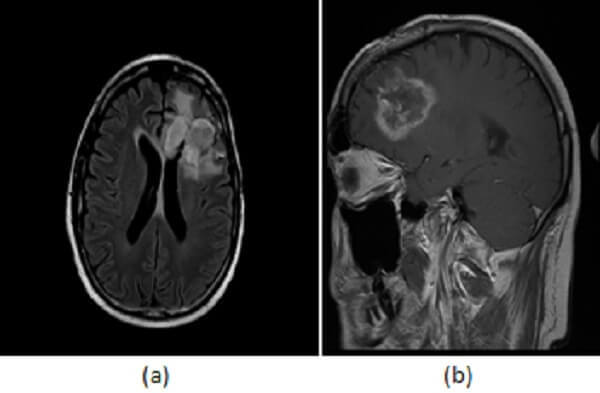
Figure 1: axial (a) and sagittal (b) post contrast T1 images demonstrate a left frontal lobe mass in the region of prior tumor resection. The mass has irregular rim enhancement and vasogenic edema making it concerning for tumor recurrence. (c, red arrow) axial image from an PET CT however demonstrates decreased FDG uptake within and around the mass suggesting that it lacks metabolic activity, which would be unusual for recurrent tumor.
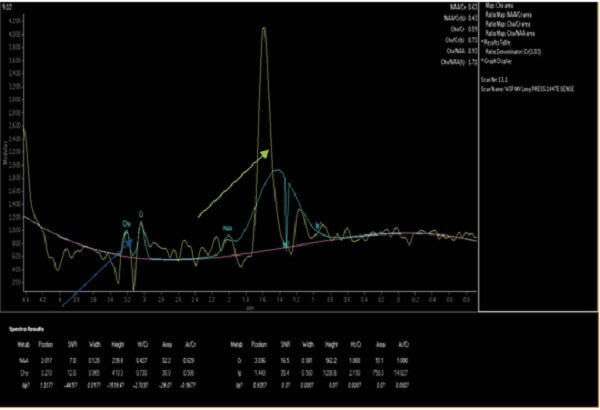
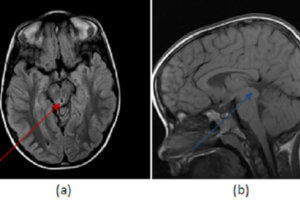
Figure 2: MR spectroscopy of the lesion demonstrates decreased choline to creatine (blue arrow) and increased lipid to lactate ratios (green arrow). The imaging findings together favor radiation necrosis over recurrent tumor.
However, in addition to damaging tumor cells, radiation also damages normal surrounding brain parenchyma. Radiation necrosis results when radiation damages the vascular endothelium of normal brain parenchyma, leading to thrombosis and ischemia resulting in the confluent coagulative necrosis of white matter. This necrotic white matter can be symptomatic and often simulates tumor recurrence.
Clinical and conventional imaging findings can suggest the diagnosis of radiation necrosis, but they are not as specific as advanced imaging modalities like PET-CT, spectroscopy and CT perfusion. Radiation necrosis is a delayed response to radiotherapy, typically occurring more than 3 months after the initial therapy. A “swiss cheese” or “soap bubble” appearance to the enhancing mass, occurrence of lesions remote from the original tumor or at sites where tumor was not previously present, and disproportionate involvement of the periventricular white matter all favor radiation necrosis.
Advanced imaging modalities add significantly to this diagnostic dilemma. On PET-CT Radiation necrosis is hypometabolic relative to contralateral white matter, whereas tumor has increased FDG uptake/metabolic activity. Similarly, CT perfusion demonstrates decreased regional cerebral blood flow to radiation necrosis but increased blood flow to recurrent tumor. Finally, the MR spectroscopy of radiation necrosis is characterized by decreased choline/creatine and increased lipid/lactate ratios, findings that are reversed in recurrent neoplasm.
Distinguishing radiation necrosis from recurrent tumor remains a challenge despite advances in neuroimaging. To further complicate matters, these conditions often coexist which can result in inconclusive imaging. Biopsy may be required to distinguish recurrence from radiation necrosis, but advanced imaging modalities can help select the biopsy target by identifying regions of the mass with the most suspicious features.